Knowledge Bank
Common questions regarding the Concentric digital consent application from clinicians and deploying teams, divided into the broad themes of template structure and content queries, process queries, and device queries.
Consent template structure and content
Can Concentric be used for all consent form types (i.e adults, children and adults without capacity)?
Yes, Concentric is used for all core consent to treatment scenarios. Depending on the scenario different consent options will be presented - for example for an adult patient consent form 1 (adult with capacity) and consent form 4 (adult without capacity) options will be presented, and depending on the selection different information will be required.
Our consent form 4 guide describes how a best interest decision / consent form 4 is completed, including video walk through. Concentric is also used for consent on behalf of a child or young person (consent form 2). Our consent form 2 guide walks through this process and the differences between a consent form 1 and 2 in Concentric.
Can I add new content, or edit existing content?
Yes, content updates can be requested via the 'Content request' link in the account menu within Concentric or via the healthcare organisation, and follow the process outlined in our process for content template updates page
At an individual patient level templates can be modified by the clinician to ensure that the information is appropriate for the individual. If no template is available, a blank template can be used.
Is anaesthetic consent managed within Concentric?
Concentric focuses on the surgical/treatment consent element rather than the documented anaesthetic consent which has traditionally ran in parallel, often on the anaesthetic record without a patient signature.
Some anaesthetic information is shared within Concentric - such as likely anaesthetic approach - with this information selected by the clinician as part of the consent process. This information includes links out to trusted resources such as the RCOA (Royal College of Anaesthetists) patient information.
A separate consent episode can however be done for the anaesthetic technique - e.g. spinal anaesthesia - if wished.
Additional consents - what are they and what are they not?
‘Additional consents’ within Concentric are linked consent modules done alongside the primary treatment or investigation consent. Our additional consents page summarises what they are, shares some examples, and how to request new additional consent modules.
What they are not used for
We sometimes get asked whether it’s appropriate for things like “+/- urinary catheter” or “+/- drain insertion” to be added as additional consents.
Within Concentric these are included within the risks section. This is based on user research with patients which showed that many were confused by the distinction between some things that were listed as complications and some things that were listed as additional procedures. It made more sense to patients to understand them together - i.e. this could happen, and therefore we might need to do x/y/z.
The second reason for this model is that these sort of small +/- additional procedures are often not separate to the treatment consent, they are intra-operative decisions made based on the context at the time in terms of giving the best patient outcome. Therefore, they are often not things a patient can opt out of in the way a Concentric additional consent would allow them to.
Who can be a ‘Responsible Clinician’?
Within Concentric, every episode is assigned a 'Responsible Clinician'. This is based on recommendations from the Francis Report (2014), and the subsequent guidance from the GMC and Academy of Medical Royal Colleges (AoMRC).
The guidance outlined that each episode of care should be under the care of a named individual - termed either a ‘Responsible Consultant’ or ‘Responsible Clinician’. It also stated that patients should be made aware of who their Responsible Clinician is.
Who can be the responsible clinician?
Guidance from the AoMRC: “In most circumstances the Responsible Consultant/Clinician will be the consultant doctor. However, in some cases it may be another senior doctor (e.g. a Specialty Doctor) with the right level of competence or another clinical healthcare professional.”
This is the reason that a clinician can assign themselves as the responsible clinician within Concentric, where appropriate, even if they are not of consultant grade.
Are non-English languages supported within Concentric / Can information be translated for patients?
Yes, the Concentric clinician and patient applications are both compatible with standard browser translation tools. Depending on the browser this enables automated translation of hundreds of languages and includes translation of both the Concentric Ontology information and cricially also any custom elements or notes added by the clinician for the individual patient. Automated translation has come a long way in recent years with vast investments made to improve quality and reach parity with human translation, including for translating medical content.
Browser translation is supported through the application, including both the treatment information and the steps for patients to go through to give their consent. Below are some examples of how to change the language on a web page:
- For Google Chrome
- For Apple Safari
- For Microsoft Edge
- For Mozilla Firefox
It is important to note that a translation does not replace the need for an interpreter as part of having a ‘meaningful dialogue’ consent conversation - as is required by GMC guidance and medico-legally. Details of the interpretation (interpreter details and notes regarding the conversation and/or interpretation) can be documented within Concentric as part of the consent flow.
Can Concentric be used for oncology?
Yes, Concentric is used across all specialties, including across both radiotherapy and chemotherapy. The consent process is very similar for oncology as it is for procedures, with small modifications:
- appropriate wording is used, so for example ‘your treatment’ is used instead of ‘your surgery’,
- things that are irrelevant - such as consent statements around discussing anaesthetic plan – are removed.
We use the CRUK (Cancer Research UK) and RCR (Royal College of Radiologists) templates as the basis of our oncology consent templates, and work with both organisations. Both organisations have mechanisms in place to inform the Concentric team of the release of new templates or updates to templates. As with all other content, these templates can be modified locally should you wish.
RCR - We were the partner organisation with the RCR for the national standardised radiotherapy templates project.
CRUK - We were named as a supplier in CRUK’s econsent guidance (see page 24-25). There are a large number of CRUK templates and the clinical team have been adding these as templates in Concentric based on their volume of use. There are some that do not have their own template within Concentric as of yet, and a generic SACT template is used in this example.
Can we change the likelihood categories to match the ones our Association / Royal College use?
Based on user research with patients we made the decision to present risks according to both timing (when in the patient journey they may happen), and likelihood. 3 groups are generally a good approach for these sort of thing - giving enough granularity to differentiate, without presenting a confusing and difficult to digest number of options. Hence we landed on 3 categories for timing (immediate, early, late) and 3 for likelihood (common, less common, rare) - you can see the definitions for each in Concentric.
A challenge here is that there has never been an agreed standard in terms of how risks are presented, which means that each team / company / association / Royal College who have prepared consent forms or patient information have come up with their own approach and definitions.
This presents complexity for patients who have to work out the context for how a risk is being described, which may be quite different to how things were described when they had a previous procedure by a different specialty.
For this reason we are attempting to introduce some standardisation - we see no good reason for why risks should be described differently depending on the specialty - and are in a good place to drive standardisation as a platform that is used across all specialties and across many healthcare organisations.
Are Consent Form 3’s used in Concentric?
Consent form 3’s are not used within Concentric as this is simply another version of ‘adult with capacity consent’ - i.e. the same as a Consent Form 1. Adult with capacity consent is labelled as a Consent Form 1 within Concentric simply as this is what it is usually known as by clinicians.
For paper consent forms where no dynamic logic can be applied, Consent Form 1 and Consent Form 3 options were required to differentiate between procedures where general anaesthesia / sedation were used and those where a consciousness would not be impaired - this logic and the sharing of appropriate anaesthetic information is managed within Concentric depending on clinician selections in clinician view.
Can Concentric be used for research consent?
Concentric is, at least currently, a digital consent to treatment application - i.e. consent to any procedure, treatment, or operation (Concentric’s current model can also be used for most invasive investigations).
There are research consents that are done as part of Concentric, but these are all related to the core treatment consent - they are an additional consent in parallel to the treatment consent.
Currently, we do not offer the functionality for research protocols that are not associated with a treatment consent to be completed within Concentric and would advise using a different platform for this. (We do get asked about research consent commonly, and therefore it should be of no surprise that we expect to expand into this area, but do not have a set timeframe for this work currently).
Digital consent process
What support is there for clinicians getting started with using Concentric?
A getting started guide is shared with each new clinician user, embedded as a link within the account activation email. In some circumstances the getting started guide is managed by the healthcare organisation within a training portal, and in others the onboarding information is hosted by Concentric. They can all be accessed via the getting started section of the website.
The getting started guide usually covers the rationale for moving to digital consent, stepwise guide including test patient details, information about local integration, and local support processes.
Something isn’t working! What shall I do?
If there seems to be an issue with Concentric or how it is communicating with other systems within your healthcare organisation these should be reported to your organisation. The most appropriate reporting route or contact details are shared within your healthcare organisation's onboarding guide.
Where there is an issue with Concentric itself (rather than any local integrations) our status page will reflect the status and share any status updates. Each organisation should have a business continuity plan in the rare circumstance that Concentric cannot be used for consent.
Can a witness sign on behalf of a patient?
When a patient is physically unable to sign their consent (for example patients with severe burns to both hands) it is appropriate for a witness to sign on their behalf. This is currently not supported within Concentric, and is the only scenario outside of business continuity processes where we advise clinicians to use a paper consent process. We plan to add witness signature functionality to Concentric in a future update.
Can I modify consent information once consent has been given?
Currently, when consent is given it enters a locked state where only certain actions can occur, such as confirming or revoking consent. Therefore, to make changes such as changing the procedure name or risk profile – anything requiring more than a note at point of confirmation of consent – a new consent episode is needed.
We have received feature requests for the ability to edit consent information after consent has been given. We are supportive of this, with appropriate logic around re-signing etc., but do not currently have a timeframe for delivering such functionality.
Can the template checkboxes be selected by default?
Once a treatment is selected in Concentric the default position is that quick-select template options for each of the different elements (indications, risks etc.) are presented for that treatment. The clinician then selects the relevant options for the individual patient, either from the template options or custom additions, including consideration of any material risks. This approach is taken as the default approach in order to support best practice and the medico-legal demonstration that the individual patient has been considered by the clinician.
We know however that there are some scenarios where the same information is selected almost every time, and in many of these scenarios the balance of benefit is towards quicker selection of the standard options, especially as in many of these cases there is very limited time with patients due to system pressures. We are actively considering approaches, whilst still enabling personalisation to the individual.
Do clinicians get notifications of remote consent being given?
Within Concentric a clinician can review the state of any episode by searching for the patient. Currently we do not provide functionality for clinicians to receive automated notifications when consent has been given. There are some situations where we believe this may be valuable to users - for example where a clinician does a small number of large cases, or sometimes in private practice - but for many this would just create notification noise either in-app or via email.
Can patients revoke consent within the patient application?
Consent can be revoked for any episode where consent has been given (either first stage consent or following confirmation of consent). Revocation can only be done on a clinician login, not via the patient application. This is analogous to how it would be managed historically with paper consent, and is appropriate for 2 reasons:
- Revocation of consent is not usually an end point - it is usually a trigger for a conversation around alternative approaches. Managing revocation of consent via the clinician application ensures that no revocation events are missed.
- There is a risk that revocation could be completed at the last minute by the patient if it were available via the patient application. This could lead to a situation where the clinical team are not aware that revocation has occurred (for example an updated ‘revoked’ consent form may not have arrived into the EHR), leading to medico-legal complications should the procedure proceed.
Can patient feedback be collected via Concentric?
There is an automated mechanism that can be used to send a survey link out to patients via email shortly following consent. This can be a Concentric hosted survey or one that the organisation prepares (sometimes we see organisations like to use the same survey platform for all their surveys). There is also the option to add a Shared Decision Making (SDM-Q-9) questionnaire to the survey.
Patients also have the option to add free-text feedback at any point within the Concentric patient application. Any such patient feedback for patients from your organisation are visible within the Concentric admin application.
Can multiple clinicians be editing consent information at the same time?
No, in the unlikely event that someone is editing that consent episode at the same time an error message will show to warn that the consent information you are seeing has been further edited elsewhere and will block further edits until you refresh the page two bring all versions back into sync.
Will the clinician’s email address / mobile number be shared with the patient?
No, the communication to the patient comes from Concentric, with the healthcare organisation’s branding, rather than from any clinician's email or mobile number, and so clinicians email addresses and mobile numbers are not made visible to patients. Read more about patient communications in Concentric.
Can we record pregnancy status or a pregnancy test result in Concentric?
No, we do not believe the consent form is the correct place to document this information.
Whilst deploying Concentric we have seen that a minority of organisations have had a pregnancy status section within their paper consent forms. These are sometimes a question regarding whether the patient may be pregnant, and sometimes the documentation of a pregnancy test.
Our experience is that each time this has been explored, the organisation has reflected that the consent form, given recent context, is not the correct place for documentation of pregnancy status.
With a move away from consent on the day of treatment – as per best practice, there may be a long period of time (including longer than 9 months) between the first-stage consent documentation and the treatment. Pregnancy status is only relevant, in this context, at the point of treatment.
Following UK NatSSIPs 2 national guidance, pregnancy status should be elicited as part of the pre-assessment checks for all major procedures, and minor procedures where radiology is required or anticipated during the treatment. This forms part of the pre-operative assessment process (which happens more consistently in the days or short number of weeks prior to treatment than the consent process), with confirmation of status as part of the ‘Sign-In’ check.
A digital patient record provides the opportunity to ensure that information recording is not duplicated, both to increase efficiency but also to remove the risk of conflicting information and subsequent error. Pregnancy status has broader applicability that simply on the consent form, and therefore should be documented in the single most appropriate place.
Where treatment is being undertaken during confirmed or possible pregnancy this should form a part of the consent record. The addition and modification of information within the consent information to reflect the context – for example risks to the fetus or increased maternal perioperative risks – should be ensured, and can be managed within Concentric.
Device queries
Is there a Concentric app that I can download?
We don't have native applications, iOS or Android, but many clinicians add Concentric as a homescreen shortcut. Concentric is a web application, and clinicians access it on a wide range of devices, running various operating systems. As a web application, there is nothing for users to download before they can use Concentric – whether they are patients or clinicians, and users are able to easily switch between devices to access Concentric without it impacting their experience.
From a develoment perspective, being a web application means we only need to maintain a single application, supporting us to regularly deploy improvements to Concentric (take a look at our product updates) whilst not requiring users to install updates. It also means that we can utilise browser-based accessibility functionality, such as translation of consent information into multiple languages, and the use of screenreaders, without additional development effort.
Homescreen shortcuts are easily added. For example:
- On iOS Safari, whilst on Concentric, click 'Add to Home Screen' in the browser's 'Share' menu.
- On Android Chrome, whilst on Concentric, go to the browser's menu (three-dot icon) and click 'Add to Home Screen'.
Can I check consent on an Android device without downloading the PDF?
The Chrome browser on Android devices does not include a built-in PDF viewer. As a result, to view a PDF in Chrome on an Android device, you must first download it and then open it in a dedicated PDF viewer.
In scenarios such as pre-theatre checks, the post-consent view in Concentric can be used to view details of the consent episode. You can display the full details of the consent episode by clicking 'View full details'. The patient's signature can also be expanded on its own:
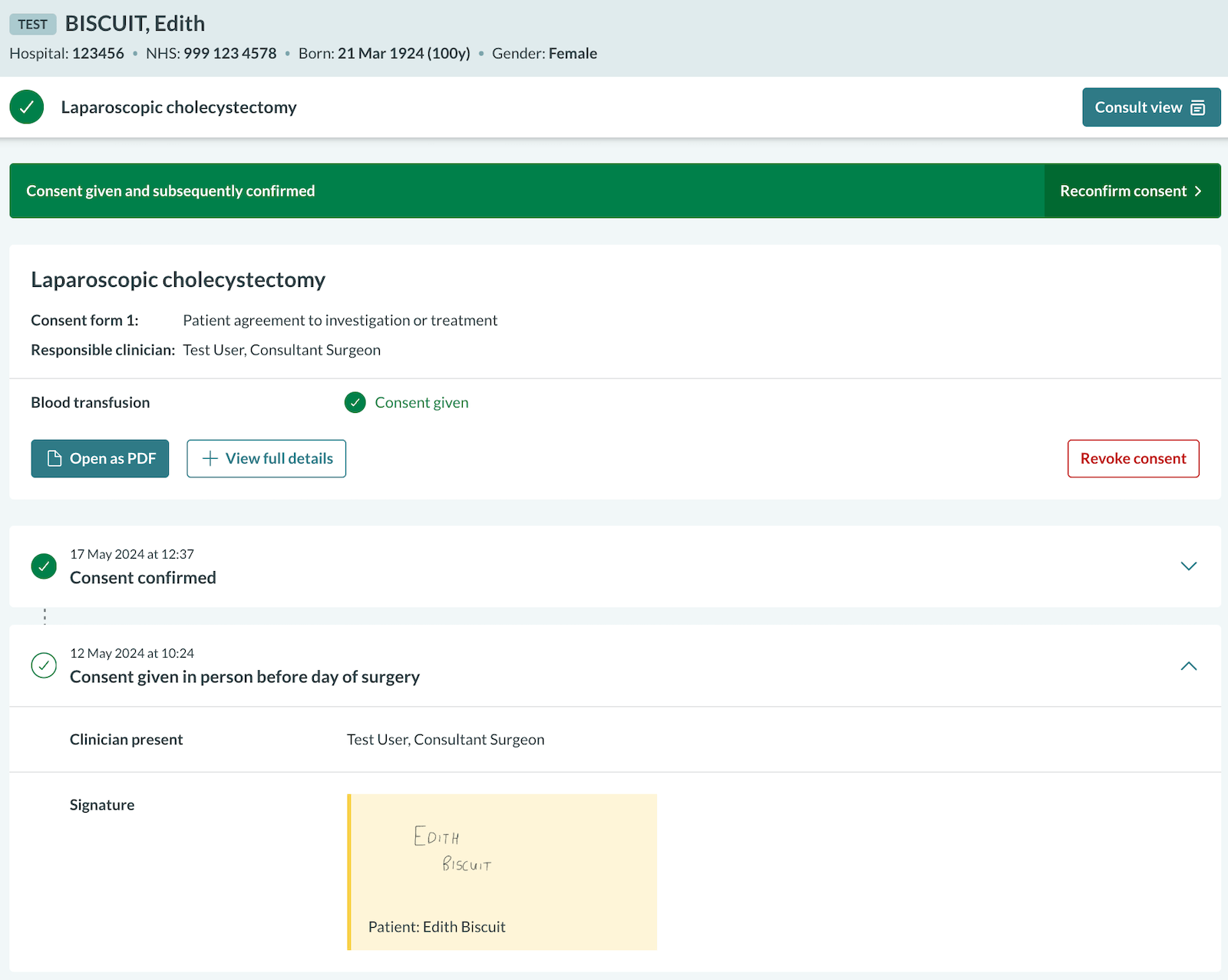
Alternatively, Mozilla Firefox can be used on Android devices, which includes a built-in PDF viewer, allowing PDFs to be viewed without downloading them.