Additional consents
This page summarises what additional consents within Concentric are, shares some examples, and how to request new additional consent modules.
What are additional consents and how do they work?
‘Additional consents’ within Concentric are linked consent modules done alongside the primary treatment or investigation consent. For example, for a patient giving their consent for a total knee replacement, the following additional consents may be presented within clinician view:
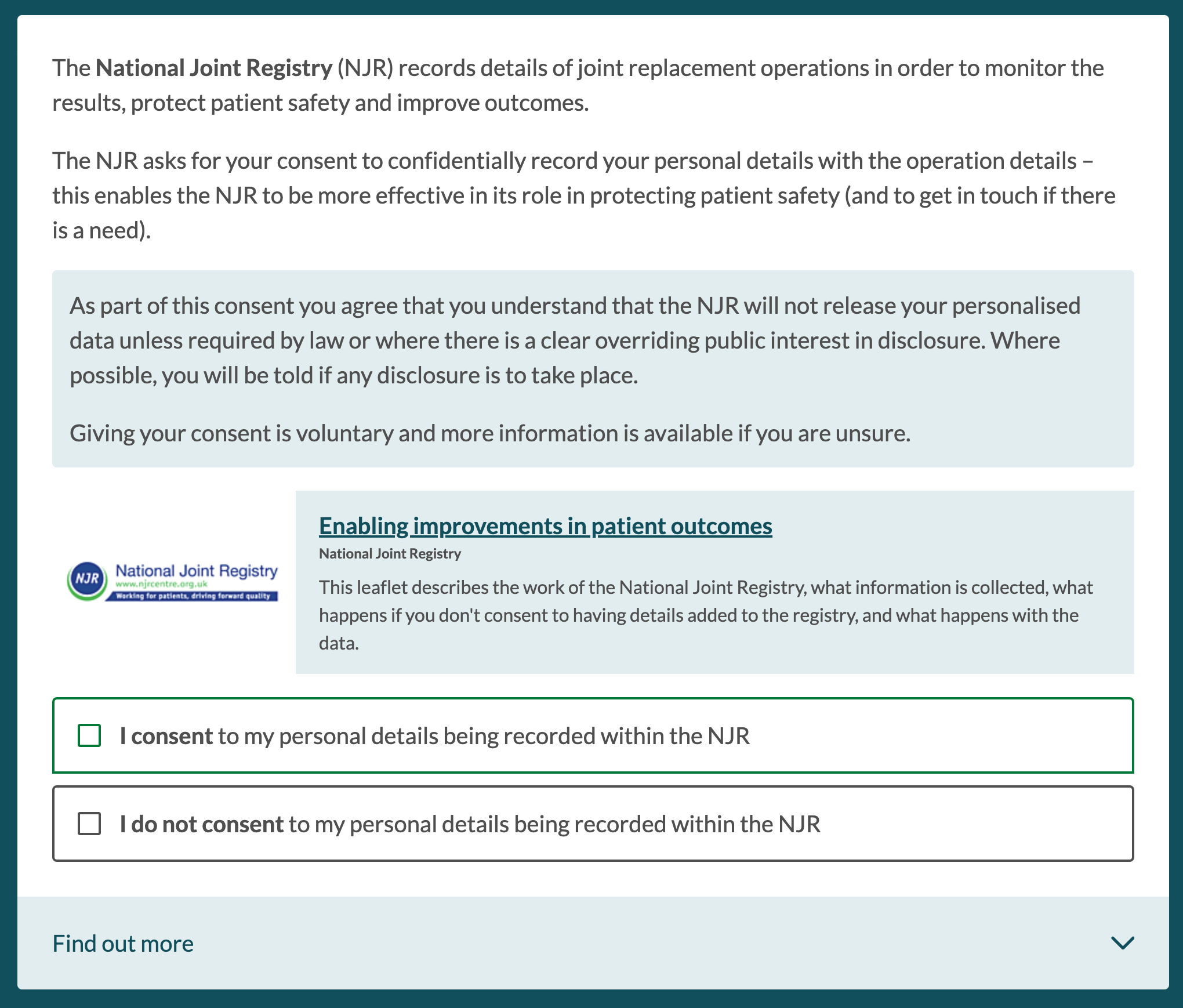
On selecting an additional consent, this is then presented within the consent flow in consult view / patient view. The information presented to patients is configured based on requirements, but will often have a structure similar to this one, for the National Joint Registry, including some introductory text, a link and/or expander for further information, and a consent statement:
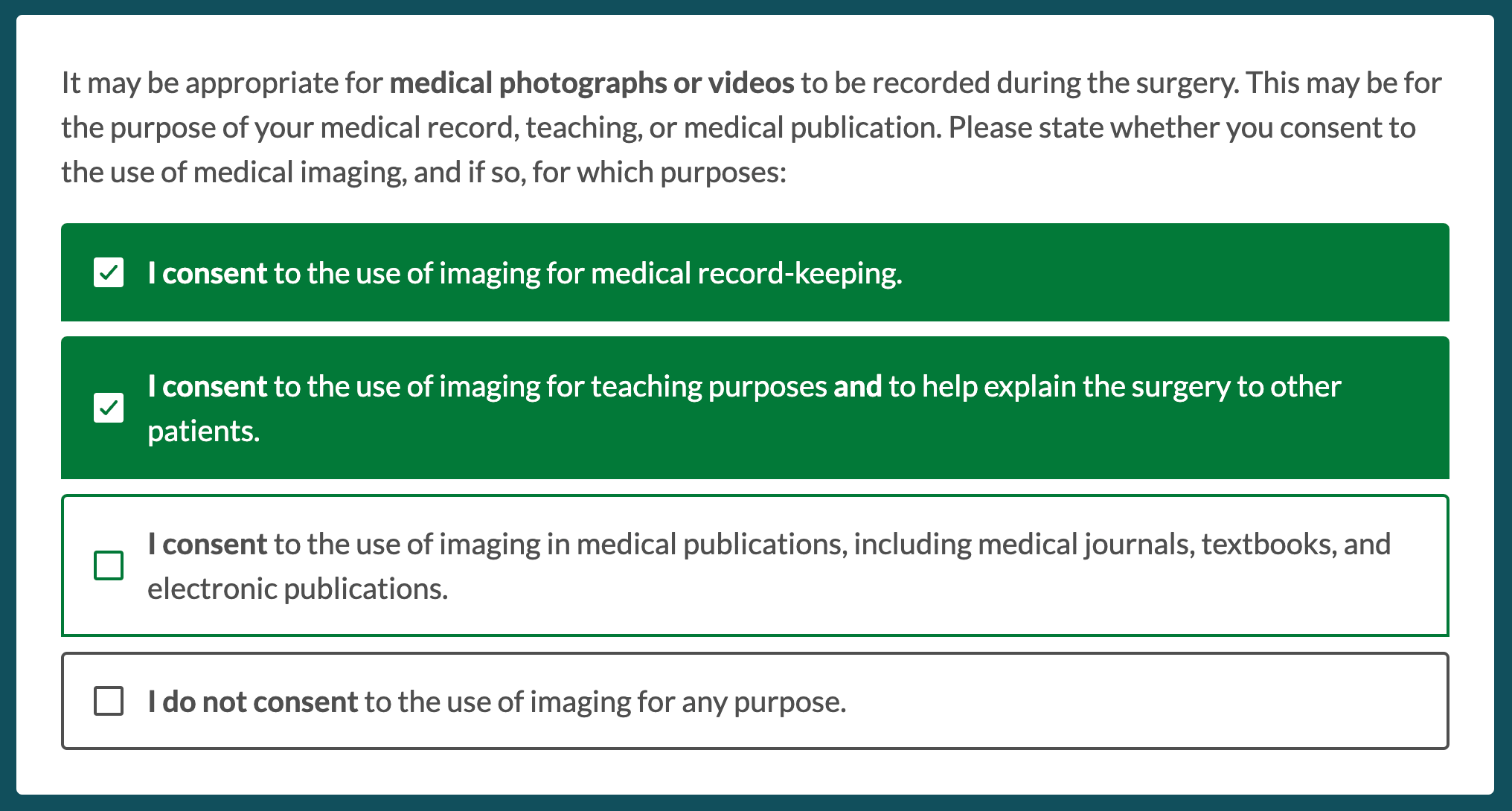
In some cases additional logic is required, for example additional questions, multiselect options, or free text entry. These can all be managed within additional consents in Concentric, for example this additional consent for intra-operative medical imaging, where consent can be given for no use-cases, one, or many:
These additional consents, and the patient’s selections as part of the consent flow, become part of the overall consent that the patient signs. i.e. There is not a separate signature section for each.
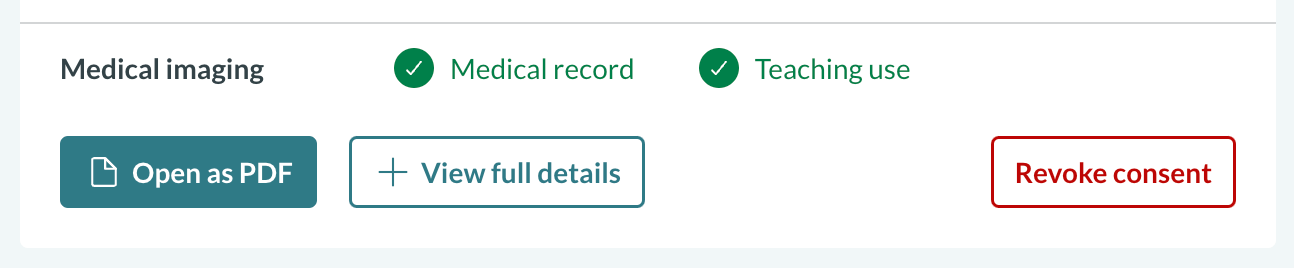
In the post-consent views in Concentric, the additional consent selections are shown, with iconography for whether consent has been given:

Where additional context is needed for each element, for example medical imaging, this is outlined:
These additional consents, with the relevant consent statements, are then shared within the consent summary PDF:

Which additional consents are available within Concentric?
The following additional consents are available nationally (UK). Individual organisations may also have local additional consents configured – for simplicity these are not listed here but this information can be requested by contacting content@concentric.health.
National Registries and Databases
- National Joint Registry +/- Beyond Compliance
- British Spine Registry
- National Bariatric Surgery Registry
- National Hiatal Surgery Registry
- National Vascular Registry
- British Society of Urogynaecology (BSUG) Database
- British Orthopaedic Foot & Ankle Society (BOFAS) Registry
- British Hernia Society Registry
Others used across a number of sites
- Blood transfusion
- Medical imaging (i.e. medical photography/video recording during the procedure for medical record/teaching/publication purposes)
- Tissue for research (this will often be modified for each BioBank, with local information)
- Sensitive disposal arrangements for pregnancy loss (this will often be modified locally due to different processes, and with local information added)
- Use of surplus tissue for teaching, quality control, and audit purposes
- Homecare Medicines Service
- CMR Surgical Registry and Versius Video
Requesting new additional consents
To request a new additional consent, please contact content@concentric.health – we will direct you as to what additional information, if any, is required.
Integrations with other systems
We do not have integrations with other specialist systems with regard to additional consents. For example, we do not currently send the information to the Registries automatically - documenting the patient’s consent in Concentric simply replaces existing paper consent forms for each.